1. What is the chemical name for Se4S4? - Answers
Aug 7, 2011 · The compound name for Se4S4 is tetraselenium tetrasulfide. It is a selenium compound in the sulphide class, and it has a red color and a ...
When people search for products or services related to your business, it's a way to improve the visibility of a business website in search engines. According to research and search pattern statistics, organic traffic is saved by this SEO strategy because it is the best way to increase the number of visitors to the site. This is done to maximize the productivity of businesses running online on the internet.

2. Se4S4 molar mass - WebQC.Org
Related compounds. Formula, Compound name. SeS2, Selenium disulfide. Se2S6, Selenium hexasulfide. Formula in Hill system is S4Se4. Computing molar mass (molar ...
Molar mass calculator computes molar mass, molecular weight and elemental composition of any given compound.

3. Se4S4 Compound Name, Molar Mass Calculation -- EndMemo
Name: Tetraselenium Tetrasulfide ; Formula: Se4S4 ; Molar Mass: 444.1 ...
Tetraselenium Tetrasulfide Se4S4 Molecular Weight, molar mass converter
4. 1,2,5,6-Se4S4 | CAS#:75935-37-4 | Chemsrc
Jan 15, 2024 · Common Name, 1,2,5,6-Se4S4. CAS Number, 75935-37-4, Molecular Weight, 444.10000. Density, N/A, Boiling Point, N/A. Molecular Formula, S4Se4 ...
Chemsrc provides 1,2,5,6-Se4S4(CAS#:75935-37-4) MSDS, density, melting point, boiling point, structure, formula, molecular weight etc. Articles of 1,2,5,6-Se4S4 are included as well.
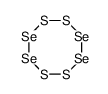
5. Is se4s4 ionic? - Answers
Sep 7, 2016 · The compound name for Se4S4 is tetraselenium tetrasulfide. It is a selenium compound in the sulphide class, and it has a red color and a ...
Se4S4 is a Molecular compound.

6. Fill in the systematic names of the following chemical compounds. Note
Sep 15, 2021 · 1. XeF: This compound contains one xenon (Xe) atom and one fluorine (F) atom. · 2. Se4S4: This compound contains four selenium (Se) atoms and ...
VIDEO ANSWER: We are writing the chemical names for the compounds. Each of these are bonds between non-metals and therefore are not ionic compounds. When we're…
7. Fill in the systematic names of the following chemical compounds
Duration: 3:09Posted: Feb 13, 2023
VIDEO ANSWER: So for this question, we are writing the systematic chemical names for each of these molecular compounds. So each of these are not ionic compound…
8. Is compound Se4S4 ionic or molecular? - MathGPT
In an ionic compound, there is typically a metal and a nonmetal that combine through ionic bonds, where electrons are transferred from the metal.
Is compound Se4S4 ionic or molecular?
9. Molecular formula Name of compound XeO2 XeF2 SeS2 Se4S4 Ni
Aug 2, 2021 · VIDEO ANSWER: Hello students, let's begin with this question. Here some of the compounds are given and we have to write down their names.
VIDEO ANSWER: Hello students, let's begin with this question. Here some of the compounds are given and we have to write down their names. So, first one is ZdO3…
10. [PDF] Compound Name
Compound Name. Indicate Type of Compound: I = ionic, A= acid, M = molecular. Write your answer here manganese (II) nitrate. I. Mn(NO3)2 bismuth (V) iodite. I.
11. Compound Type of Compound H2C204 Ionic Molecular Cr(CN)2 ...
Duration: 3:00Posted: Oct 20, 2021
VIDEO ANSWER: But in this question we are asked to classify each chemical compound. Okay, so the compounds were given. Number 1 is gen, bromide is jink bromide…